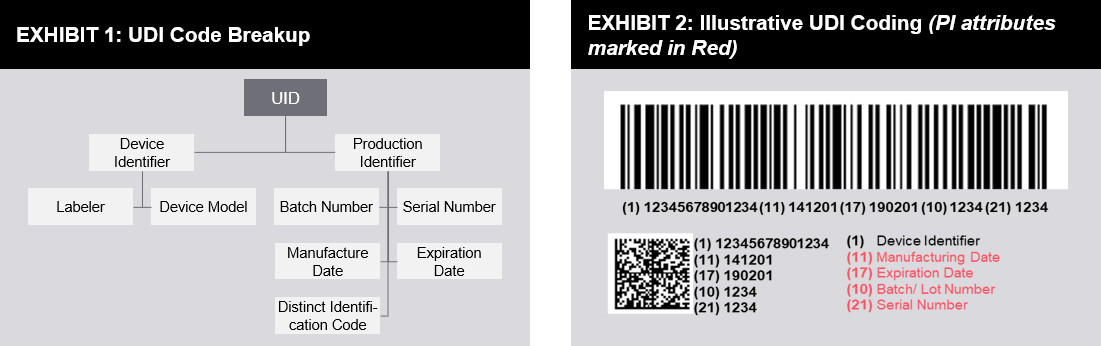
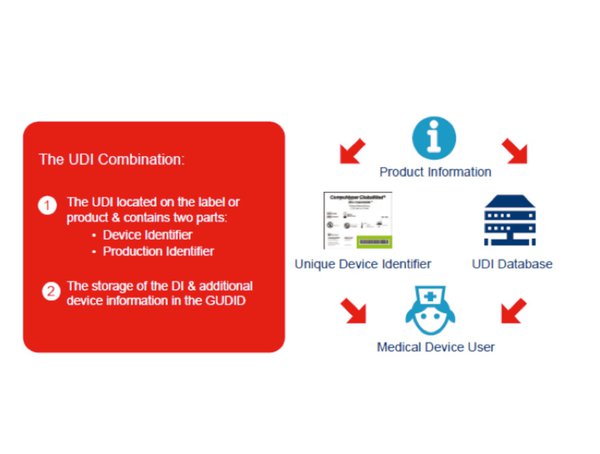
The global unique device identification database gudid contains key device identification information submitted to the fda about medical devices that have unique device identifiers udi.
Fda global unique device identification database.
A draft version of this.
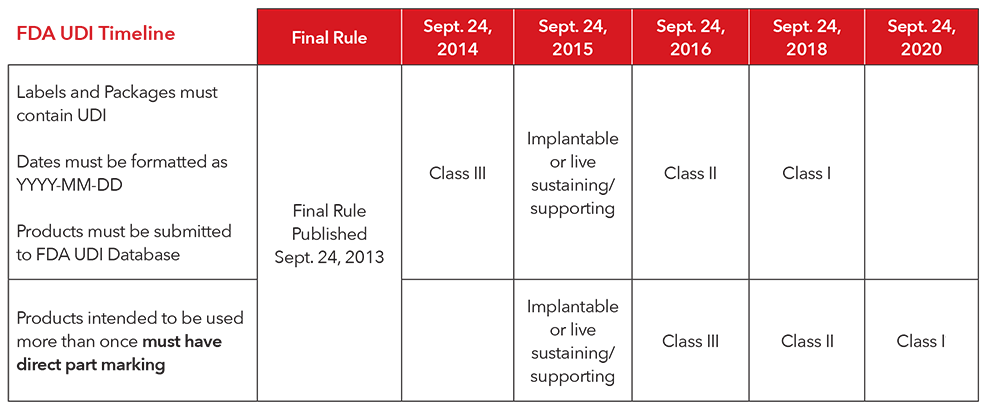
Fda established a set of compliance dates by device classification for compliance with required labeling and data submission to the global unique device identification database gudid under the.
The global unique device identification database gudid is a database administered by the fda that will serve as a reference catalog for every device with a unique device identifier udi.
Global unique device identification database gudid gudid guidance.
The global unique device identification database gudid contains key device identification information submitted to the fda about medical devices that have unique device identifiers udi.
The fda udi help desk will email you the gudid new account request document in a fillable pdf format.
Over the past year fda designed and developed the global unique device identification database gudid to prepare forthe implementation of the udi final rule.
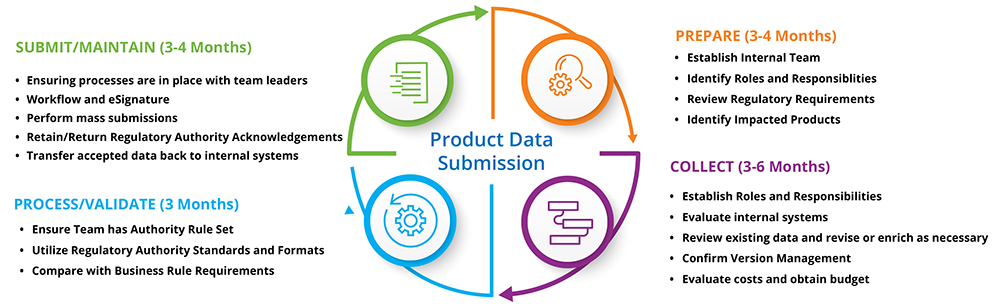
Prepare for gudid.
The unique device identifier udi should be created and maintained by device labelers based on global device identification standards managed by fda accredited issuing agencies 2 3.
Proposed rule requiring most medical devices distributed in the united states to carry a udi.
On june 26 2014 fda issued the global unique device identification database gudid.